The AID-ICU trial assessed the effect of haloperidol (antipsychotic agent) versus placebo (saline) in adult critically ill patients with delirium. Delirium is a clinical presentation of acute brain dysfuntion characterised as an alteration in attention, consciousness and cognition. This is a common condition observed among patients admitted to the Intensive Care Unit.
Results
- We assessed the effects of haloperidol versus placebo on the number of days alive and out of hospital, mortality, hospital length of stay, days alive without delirium or coma in the ICU, days alive without mechanical ventilation, serious adverse reactions to haloperidiol and the use of rescue medication. These outcomes were measured 90 days after randomisation.
- We found that haloperidol was safe to use in this population and had possible benefit in terms of improved survival.
- One year after randomisation we assessed long-term mortality, health-related quality of life, cognitive and physical function. These results are pending.
The results have provided important knowledge regarding the effects of haloperidol when used to treat delirium in critically ill adult patients. The main results have been published in the two separate publications listed below and results from the one-year follow-up will be published soon and listed here when publicly available.
Publications
Trial Sponsor
MD, Lone Musaeus Poulsen
Zealand University Hospital, Køge
lmp@regionsjaelland.dk

Useful Documents
Time Plan

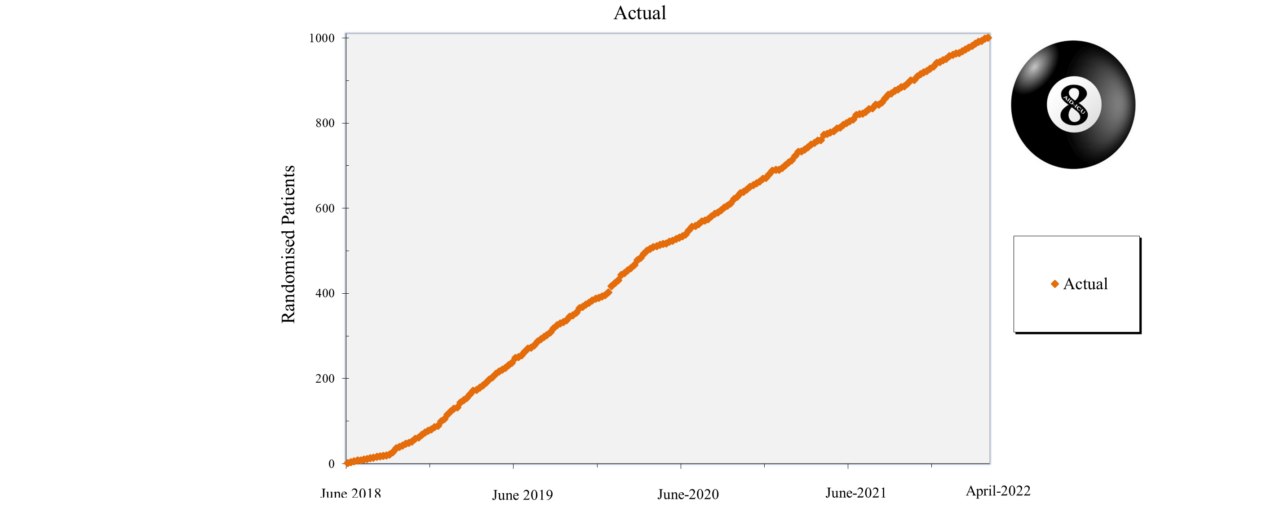
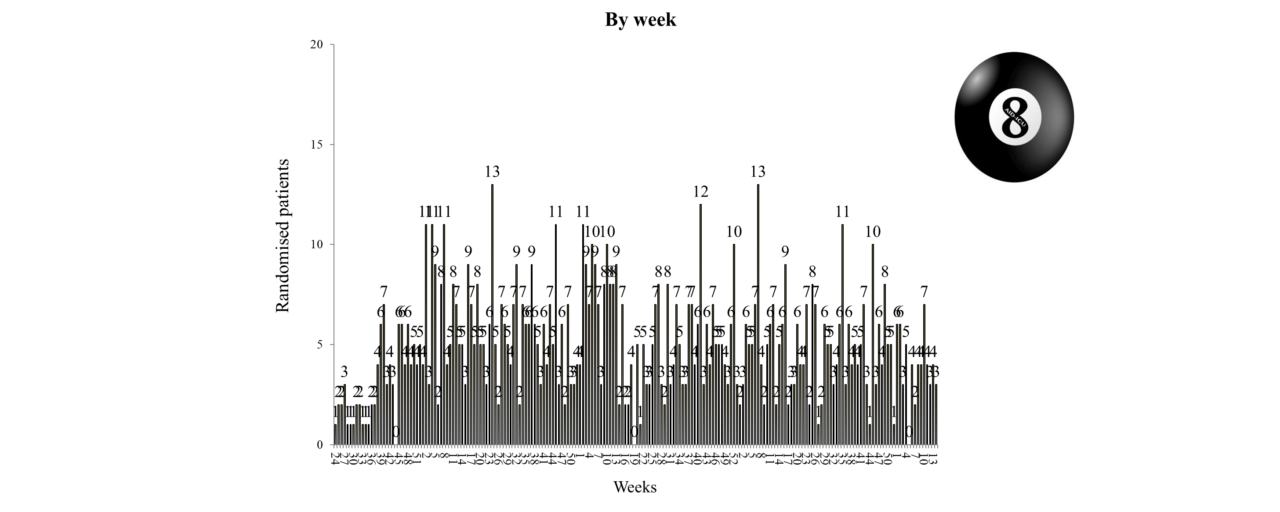
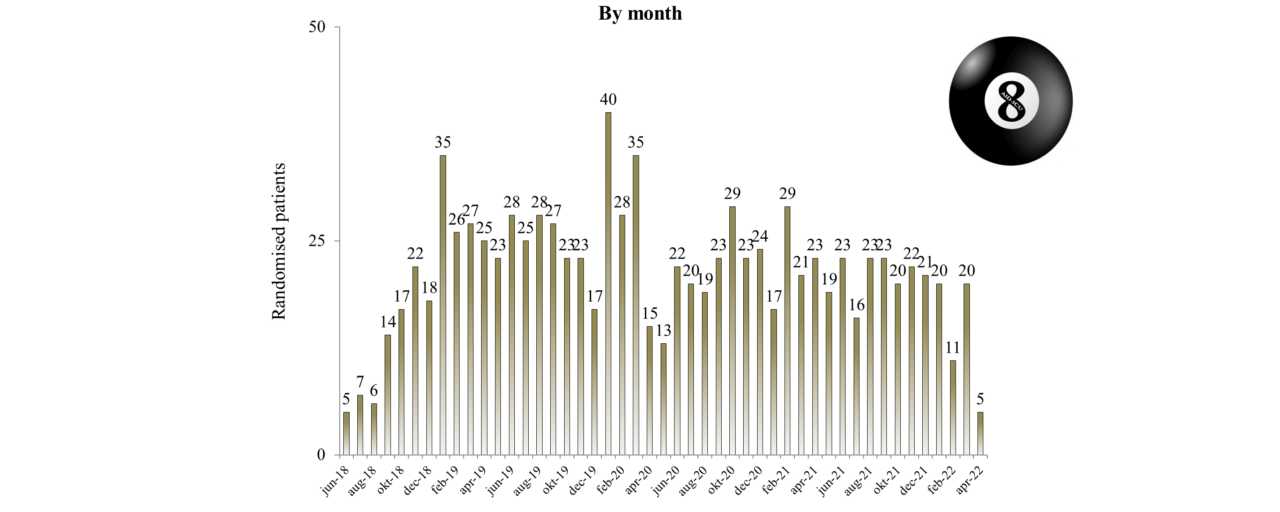
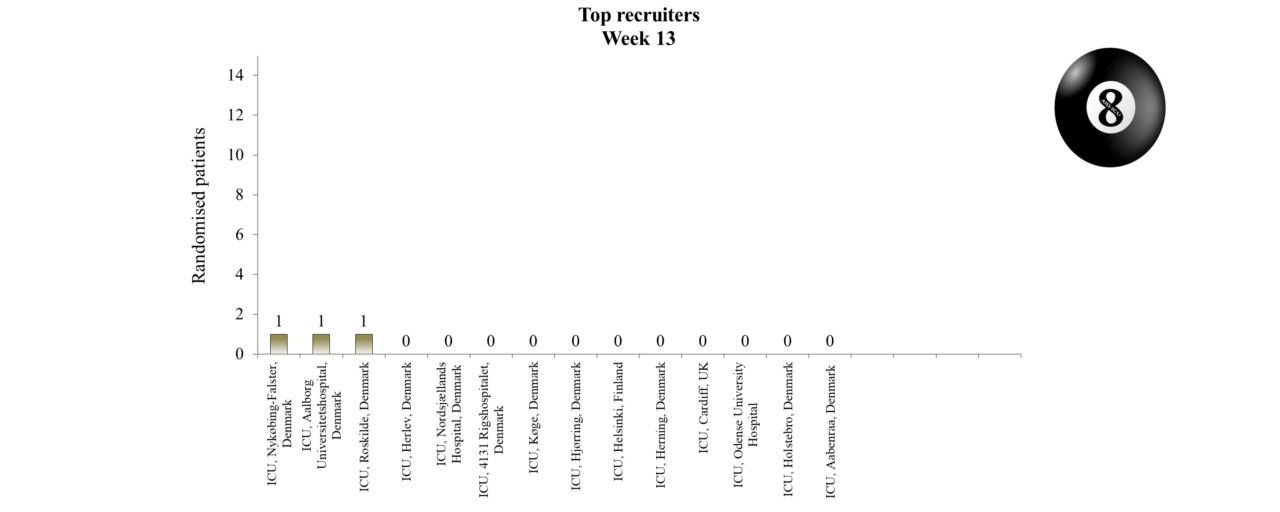
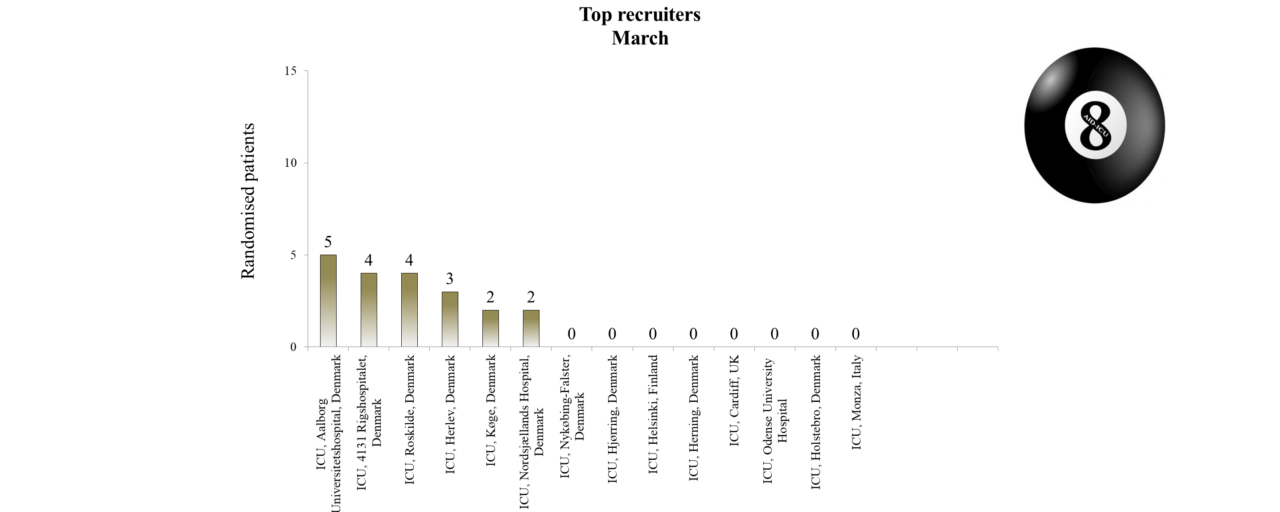
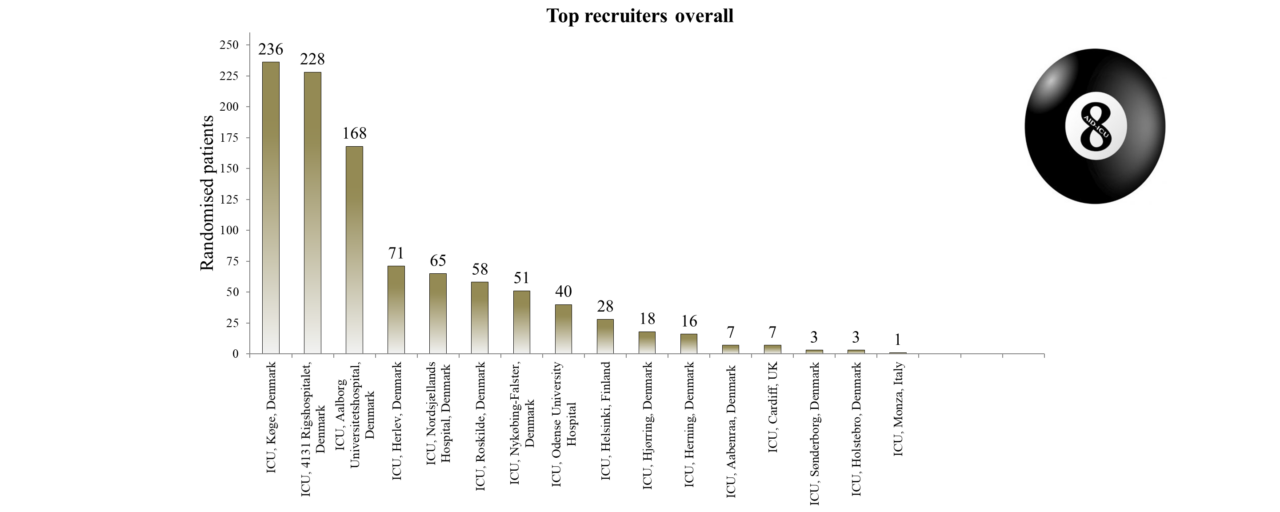
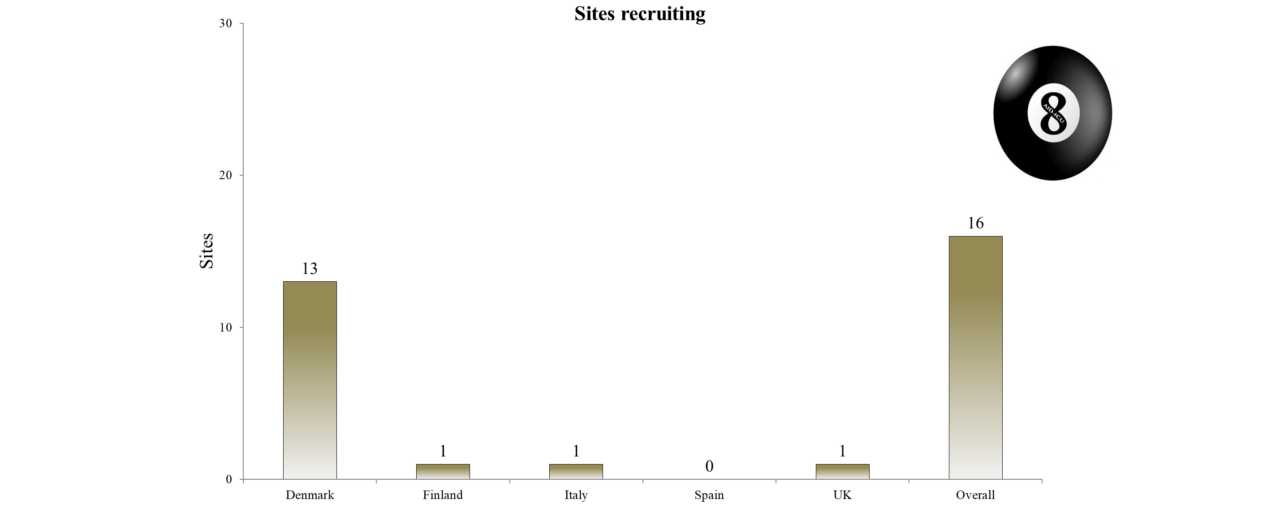
Overview of Recruitment